Abstract
Thermoset resins and rubbers are molecules that are polymerized or cured by thermal energy. After curing, these materials form a three-dimensional network of covalent connections and be converted to a non-transfiguration rigid product. Nowadays developing of simple processing methods and technological advancements makes extensive use of them in the industry.
Polymerization of the resins or vulcanization of the rubbers are exothermic reactions. On the other hand, physical properties of the thermoset bulk are dependent of reaction conversion and curing conditions. Thus the curing reaction should be a controllable and predictable process to achieve a product with tailoring and desiring properties.
Also thermal decomposition process of polymeric materials is an important and applicable process in many industries such as flame resistance for use in the aerospace industry such as spacecraft, rockets, missiles and etc. thermal decomposition process of polymeric materials causes the three-dimensional network of covalent connections break and lots of released gaseous product with a porous char mass remains.
Therefore, we need to be able to model and simulate curing and decomposition process of polymeric systems so that we can offer a mathematical equation that predict and model these important reactions.
The most common methods for investigation of curing Kinetics of resins, vulcanization of rubbers and decomposition of polymeric systems are DSC test, TGA test and ODR test respectively. Kinetics models and calculation path of all of these methods are similar and only their test methods are different.
Keywords: activation energy, Kinetics parameters, curing, vulcanization, decomposition, gel time, n-order, autocatalytic, DSC, TGA, ODR, Kissinger, Ozawa, Friedman, Modeling, Simulation, rubber, resin, polymer
Introduction
Curing Reaction
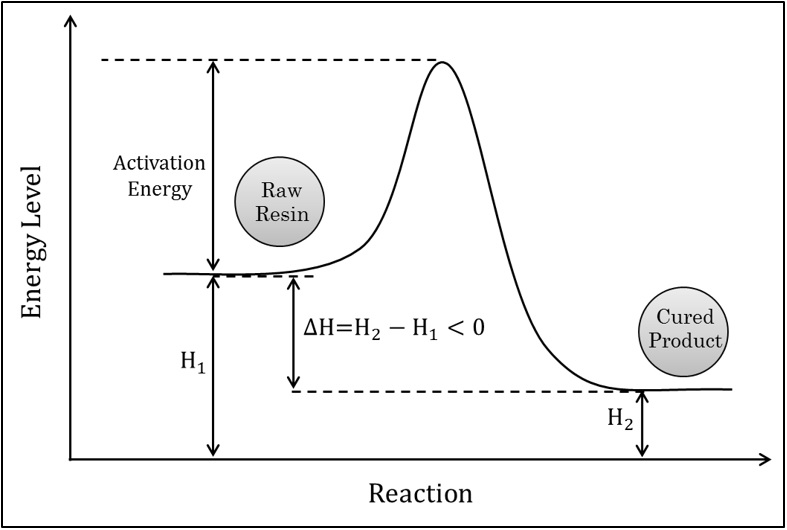
Curing reactions of thermoset resins and rubbers produce so much thermal energy therefore curing system of these materials is called exothermic reactions however the onset of chemical reaction of these materials need to thermal energy (Δ) for passing from activation energy peak. The picture shows schematic diagram of energy level variation of materials with reaction progress.
Chemical equation for the polymerization of these materials can be shown by using the following simple equation:
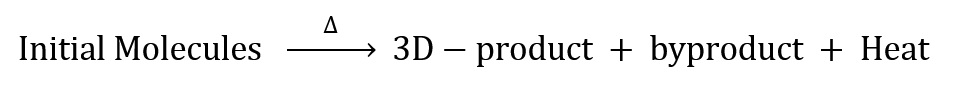
In terms of thermodynamics, these reactions are irreversible, spontaneous and have negative Gibbs Free Energy because they are too exothermic and have negative enthalpy variation (see the Figure).
At reaction of thermoset polymers unlike micro molecules, product molecular weight after start of reaction gradually increases. First dimers and trimmers form and then larger molecules from reaction of previous product were obtained. Eventually with production of a rigid and monolithic structure, the reaction ends.
Generally, the equation of reaction rate for curing and decomposition process of polymeric system is a function of temperature (T) and the extent of progress reaction (α) and is expressed as follows:
![]()
Where K(T) and f(α) are functions of reaction rate constant and conversion, respectively. Reaction constant equation is expressed as an Arrhenius form and it control reaction rate with temperature variations as:
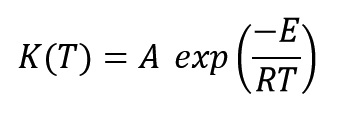
Where A, E, R and T parameters are Pre-exponential factor (s-1), reaction activation energy (j.mol-1), universal gas constant (8.314 j.mol-1.°C-1) and absolute temperature (Kelvin), respectively.
By matching these two last equations, we get:
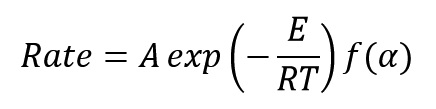
According to the equation, the rate of Kinetics reactions of a polymeric system is strongly dependent on temperature. Therefore, these reactions are extremely slow at low temperatures, so being exothermic help reaction itself provide the energy required to reaction with reasonable rate. The generated heat by the reaction at any moment help reaction rate increase in the next moment. Also functionality of curing rate to conversion means that extent of released heat from the resin during polymerization is proportional with extent of reaction progress.
In a curing at constant temperature, the heat generated at any moment in reaction is not consumed during process and only accelerated the reaction rate. Therefore, the reaction heat accumulates in material and the material temperature raises and cause reaction rate increase and more heat produce. This process is shown in the following equations:
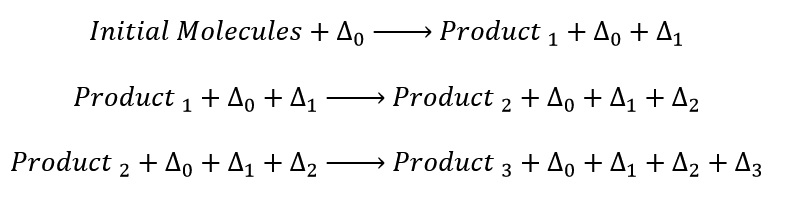
Where Δ0 is initial heat to start reaction and Δ1, Δ2 and Δ3 are heat reaction at any step of reaction. And the last reaction can be written as follows:
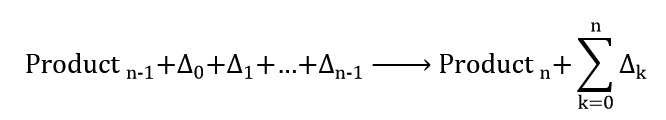
Where Δn = 0 and Productn is final product and the total amount of reaction heat is the total sum of heat during the reaction:
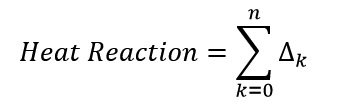
Another factor that increases the system complexity is that thermoset resins have generally low thermal conductivity and heat transfer rate in their bulk is low. This means that the generated heat by the system slowly goes out from the system and on the other hand, the reaction heat of these materials is high and reaction heat causes system temperature swiftly increase. This increasing temperature can cause expansion, deformation and distortion on product occurs. In addition, too increasing in the reaction temperature may cause decomposition reactions start and product fails.
So the reaction heat can be two conflicting effect of acting as useful factor or destructive factor. Therefore, developing of a Kinetics model that can be predicted rate of curing reaction at different temperatures help to optimize and control thermal effects of curing systems without occurrence of the damaging effects.
Reaction heat and mechanical properties
As noted at before section, a polymeric curing system releases some heat to form a chemical bond. Whatever formed chemical bond more, the amount of released heat is more. Therefore, the amount of released heat represents the amount of chemical bonds. On the other hand, much larger number of chemical bonds forms, a product with more rigidity and better mechanical properties would be obtained. Therefore, can be said that the amount of released heat of reactions shows product mechanical properties. So although the reaction heat can be destructive but no release of it means no produce a standard product.
With the help of a simple proportional relationship, a quantitative relationship between mechanical properties and released reaction heat of a curing system can be written as:
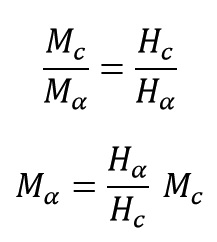
Where Mc, Mα, Hc and Hα are mechanical properties of a standard product (a completely cured product), mechanical properties of product at conversion of α, total reaction heat and reaction heat at conversion of α (0%≤α≤100%).The last equation shows that no generating sufficient heat means no completion of polymerization and no obtain standard product. In previous section was demonstrated that Hα/Hc is extent of reaction progress (α) thus the last equation can be written as:
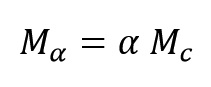
Thus an appropriate Kinetics model that can be predicted of curing rate help approximately to calculate mechanical properties of product at any conversion (α).
Conversion and Reaction Rate
In a curing and decomposition process since raw materials and final products are quite different in nature, their physical and chemical properties of the two materials would be different.
The reacting material at any conversion (α) has a property, between the initial material and the product. These properties may include dielectric coefficient, volume, density, viscosity and etc. To calculate the extent of a particular property (P), the following linear equation can be written:
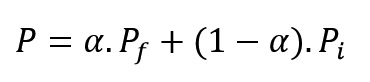
Where Pi and Pf are a particular property of initial material and final product, respectively. The above equation can be rewritten as follows:
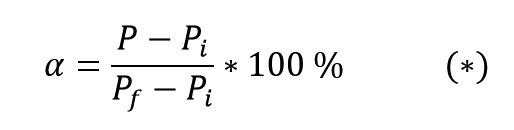
DSC test and Resins Curing
DSC machines are widely used to study the thermal behavior of thermoset resins. The test is performed by two methods of constant temperature and variable temperature that called isothermal (or Static) and dynamic DSC test, respectively. By using dynamic DSC, resin temperature increases at a constant rate and temperature response of material under range of different temperatures are recorded.
Because curing process of thermoset resins are exothermic, DSC machine record the amount of heat reaction of curing process at any moment. By using information from this test, we can calculate Kinetics parameters of curing reactions of thermoset resins.
One of the properties that change during curing reaction is the amount of released heat from the reaction. If we record the amount of released heat of curing reaction by a DSC machine, we can calculate the extent of reaction progress (α) by replacing P (in * equation) with H (Heat) as:
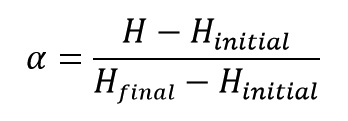
Reaction rate is obtained with deriving from degree of reaction by time:
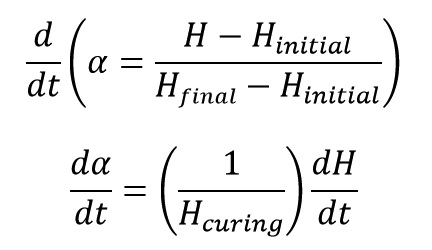
ODR test and Rubbers Vulcanization
Oscillating Disc Rheometer or ODR is a machine that by it, we can study the curing behavior of rubber materials. In this method the rubbery sample expose under the shearing Oscillating stress and the torque amount that the machine needs to Oscillate the sample is recorded.
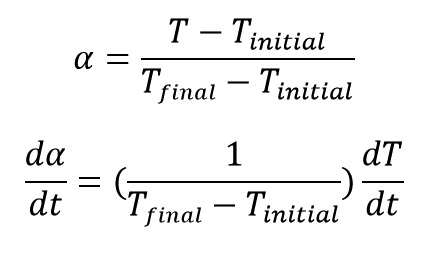
In vulcanization of rubbers we know that by increasing of reaction conversion the amount of elasticity and the stiffness of the specimen compound increases. If we record the amount of torque of the oscillating disc of the rubber sample by an ODR machine, we can calculate the extent of reaction progress (α) by replacing P (in * equation) with T (Torque) as:
TGA test and Thermal Decomposition
Thermogravimetric Analysis or TGA is a test that used for measuring of mass variations of a polymer material under high temperature over the time.
This process is implemented by TGA machine in two types:
1- Isothermal (Static) that in which, the polymeric sample weight at constant temperature is recorded as function of time.
2- Dynamic that in which, the polymeric sample is heated while temperature is changed at a constant rate.
when polymeric sample is heated decomposition reactions start. while the sample is degraded it returns to pours char and gas products. the gas products exit from the machine and the mass of the sample decrease thus the mass reduction shows Increasing of reaction conversion.
In Thermal Decomposition of polymers, we know that by increasing of reaction conversion the mass of the material decrease because Decomposition reactions cause to convert the rigid bulk of the material to gaseous product and by releasing of gaseous product, the mass of the material decrease. at the end of the decomposition reaction a constant char mass remains. If we record the mass amount of the degrading polymer material by an TGA machine, we can calculate the extent of reaction progress (α) by replacing P (in * equation) with Mass (M) as:
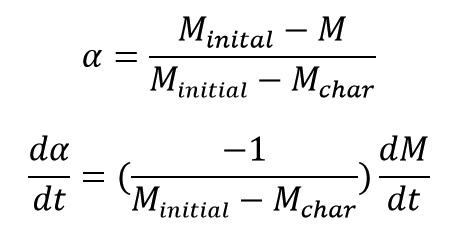
Activation Energy calculation
As we said in previous sections, the rate equation is written as:
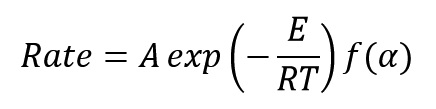
For calculating of activation energy different methods is offered. Some of these methods are depend on the chosen Kinetics model (f(α)) and some them are independent. The Advantage of Independent methods is that activation energy is determined and calculated regardless of knowing conversion function. These methods are called model-free methods. Some of these methods offer only one activation energy for all of the reaction and some others offer one activation energy for any conversion of the reaction they called isoconversional methods. In this essay we mention some important methods for calculating of activation energy:
ASTM E2890 (Kissinger) Method
Kissinger method is one of the most popular methods of activation energy calculating. By using this method, activation energy value can be obtained regardless of the conversion function. For using this method, we need to have at least three dynamic tests at different temperature rate.
Calculations trend in this method with starting from the reaction rate equation is briefly described below:
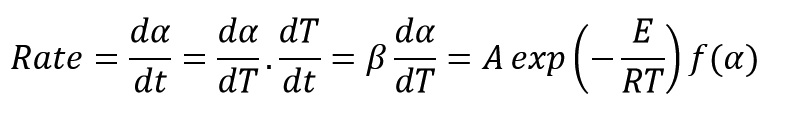
Where β is heating rate of the dynamic test (DSC, TGA or ODR).
This method is based on the fact that the curve peak temperature varies at different heating rates and curing rate has a horizontal tangent at this temperature:
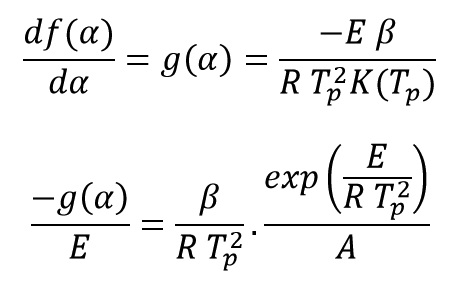
Where Tp is the exothermic peak temperature. By rearranging and taking the logarithm of both sides of the above equation get:
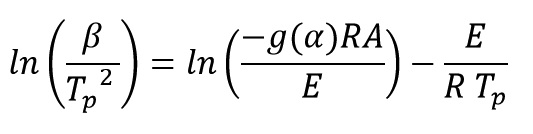
By plotting ln(β/Tp2) versus (1/Tp), activation energy is obtained from the slope of the diagram:
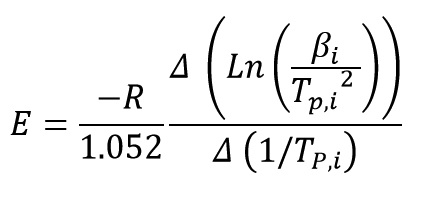
ASTM E698 (Ozawa) Method
Ozawa Method is one of the most important methods of calculation of activation energy. Calculations trend in this method with starting from the reaction rate equation is briefly described below:
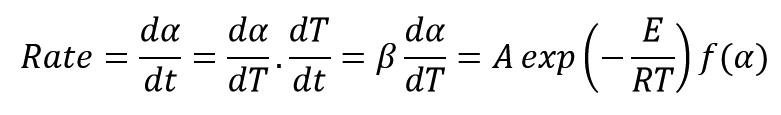
Above Equation can be written as follow:
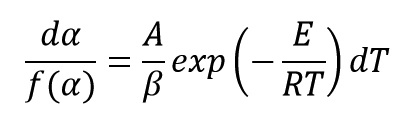
By integrating from both sides of this Equation we get:
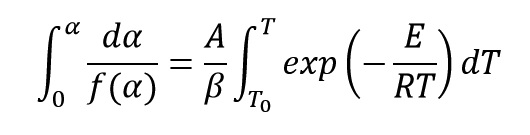
Two equations of g and P are assumed as follow:
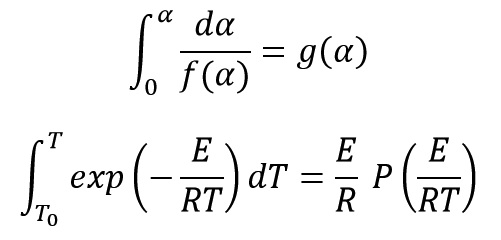
By matching three last equations, we get:
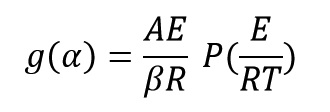
By taking the logarithm of both sides of the above equation get:
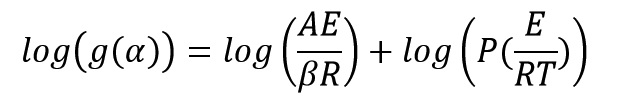
The P function could be computed by using Doyle approximation:
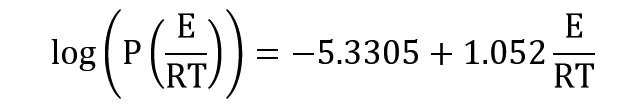
By replacing of two last equations, the following equation is obtained:
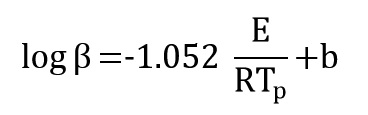
Where b is a constant value. By plotting log(β) versus (1/Tp), activation energy is obtained from the slope of the diagram:
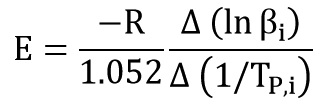
ASTM E2070
This method is for isothermal tests and needs to at least 3 isothermal data at 3 different temperatures. Also this method is model-dependent and its calculations is on the base of the autocatalytic model.
First we need to calculate constant of the reaction for each data:
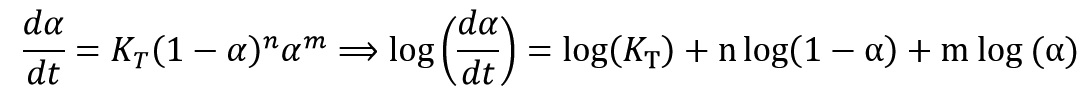
By linear regression, the constant reaction is calculated from the intercept. Then from K(T) equation:
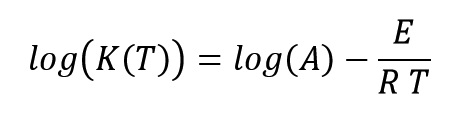
Activation energy (E) is calculated from slope of the above equation:
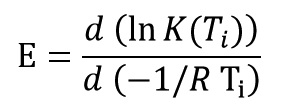
Kissinger–Akahira–Sunose (KAS) Method
This method is a isoconversional method. It means this method for each conversion value offer an activation energy and a correlation coefficient. By using Kissinger equation:
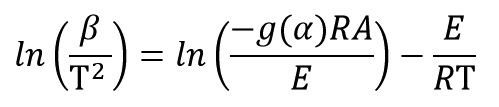
And by plotting ln(β/T2) versus (1/T) at every same conversional of data test, activation energy of each conversion is obtained from the slope of the diagram:
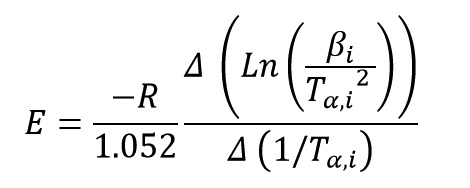
Flynn–Wall–Ozawa (FWO) Method
This method is a isoconversional method. It means this method for each conversion value offer an activation energy and a correlation coefficient. By using Ozawa equation:
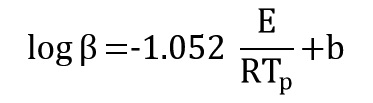
By plotting log β versus (1/Tp) at every same conversional of data test activation energy is obtained from the slope of the diagram:
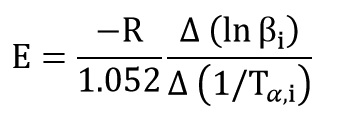
Friedman Method (FR)
Friedman method is based on the assumption that activation energy is a function of reaction progress degree and uses logarithmic form of the main equation as follow:
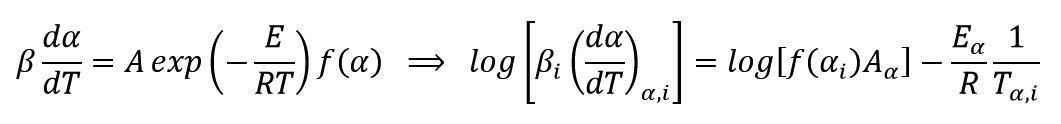
By plotting left side of above equation versus (1/T) at equal conversion (α), activation energy is obtained from the slope of the diagram:
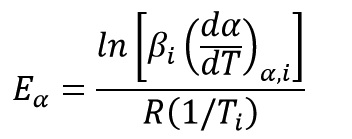
Since Friedman method for each conversion value offer an activation energy and a correlation coefficient, average of these two values are reported as final results of Friedman method.
Conversion function
After calculating of activation energy, we can calculate other Kinetics parameters. Several Kinetics models (f(α)) have been proposed to modeling of curing and decomposition systems. The most common Kinetics models are n-order model and autocatalytic model. Two mathematical equations of below show these two Kinetics models, respectively.
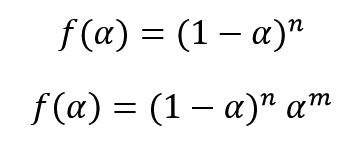
By taking logarithm from the equation of:
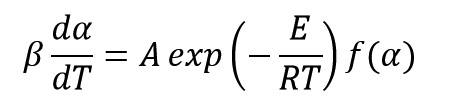
we get:
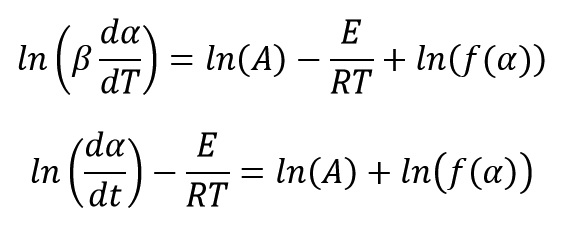
Two below Equations were obtained with replacing of model equations of n-order and autocatalytic in above equation, respectively as follow:
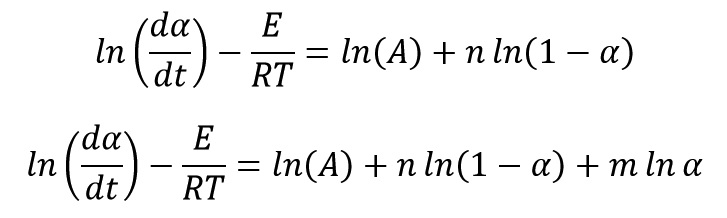
We can calculate Kinetics parameters of A, n and m by linear regression. The most appropriate model offers the best fitting with the experimental Kinetics data and give the highest correlation coefficient (R2).
Gel Time and Curing Temperature
Thermoset materials with the start of curing process create molecular bonds and form a three-dimensional and gel form structure. The time from when thermoset resin starts transformation from a viscous liquid to an "elastic gel" is called gel time. The reaction conversion that at which gelation occurs is called gel point. Conversion of gel point experimentally was obtained by observation and record of time of Adhering of a glass rod to curing resin paste while stirring. Reliability of Experimental measurements of gel time is suspicious because this process is operator dependent.
Gel time mathematically can be calculated by using curing Kinetics model.
By starting from the main Kinetics Equation and rearrangement, we get:
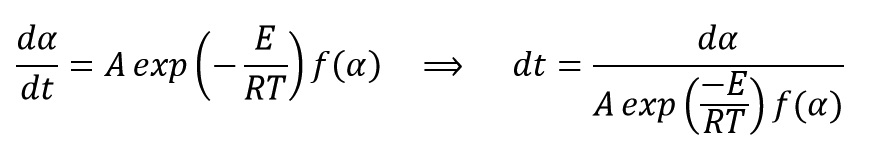
Gel time is obtained by integrating from the last above equation from curing start time (t=0, α=0%) until gel time (t=tgel, α=αgel) as follow:
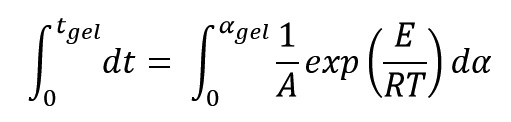
Exponential expression is constant at constant curing temperature, thus above equation finally can be written as follow:
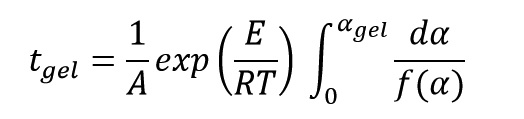
By replacing of Kinetics parameters in the equation and by solving of integral expression and by assuming αgel=20%, gel time at various curing temperatures can be calculated.
MeasSoft Thermal kinetic
MeasSoft provides a software that by using it you can simply calculate conversion and reaction rate of thermal processes and it calculate activation energy and other kinetics parameters. Also by inserting kinetics parameters you can simulate DSC, TGA and ODR tests.
Examples
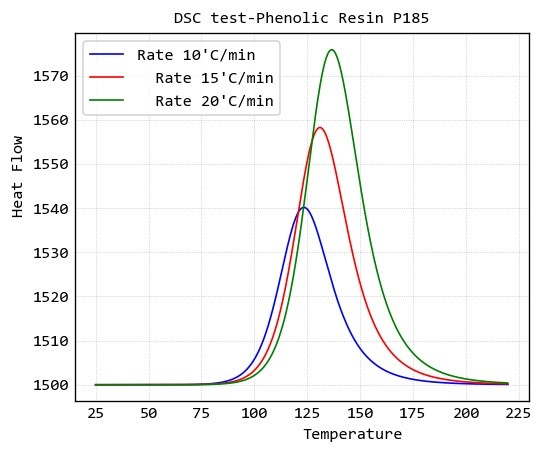
Dynamic DSC test of a phenolic resin
Dynamic DSC test of a type of phenolic resin (P185) at three heating rate of 10, 15 and 20 ̊C/min is given at below:
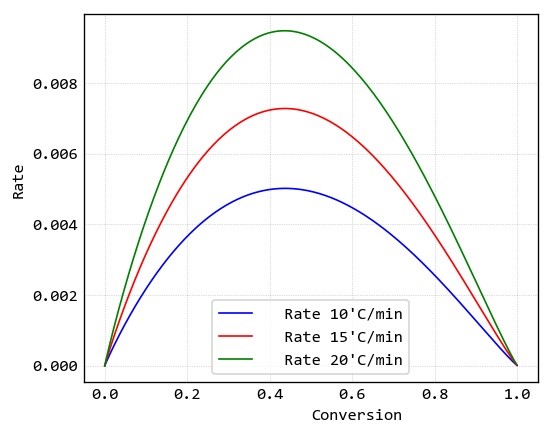
Conversion and reaction rate data of this samples are calculated by “MeasSoft Thermal Kinetics” Software as:
By helping “MeasSoft Thermal Kinetics” Software we get activation energy of this compound in isoconversional and ASTM methods as:
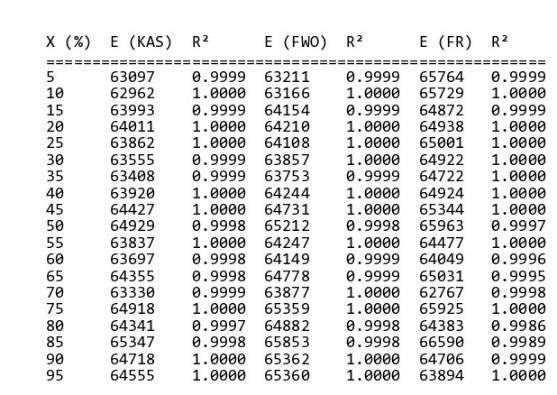

The amount of 65000 is considered as average value of activation energy of this material. also calculating of other kinetics parameters of this sample by autocatalytic is calculated by “MeasSoft Thermal Kinetics” Software as:
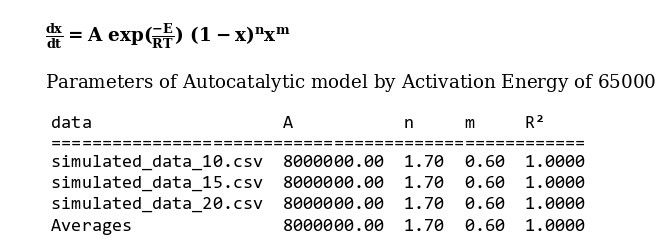
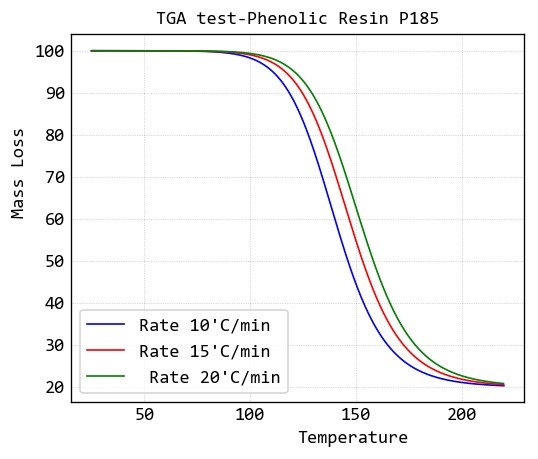
Dynamic TGA of phenolic resin
For study of degradation reaction of a type of phenolic resin (P185) Dynamic TGA test of it at three heating rate of 10, 15 and 20 ̊C/min is given in below:
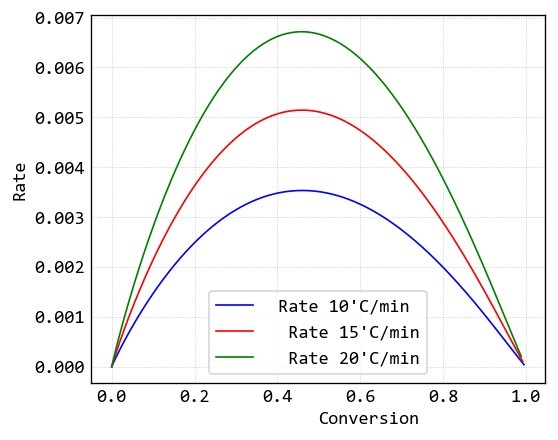
Conversion and reaction rate data of this samples are calculated by “MeasSoft Thermal Kinetics” Software as:
By helping “MeasSoft Thermal Kinetics” Software we get activation energy of this compound in isoconversional and ASTM methods as:
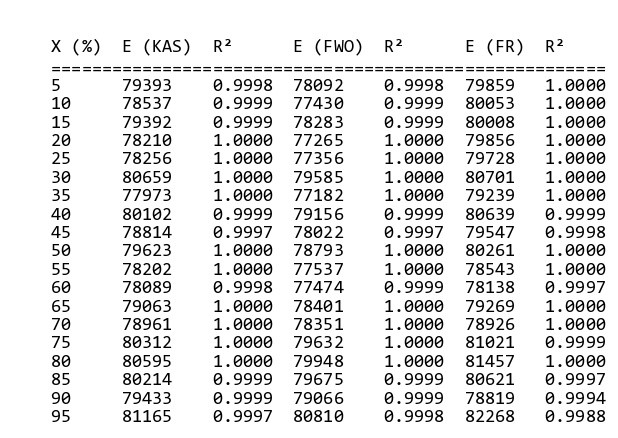
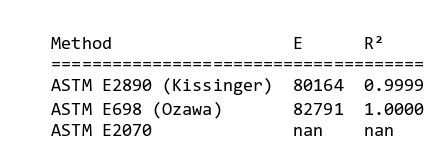
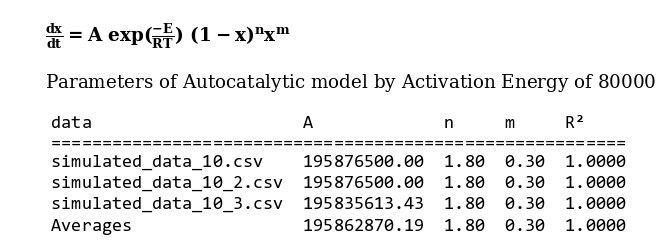
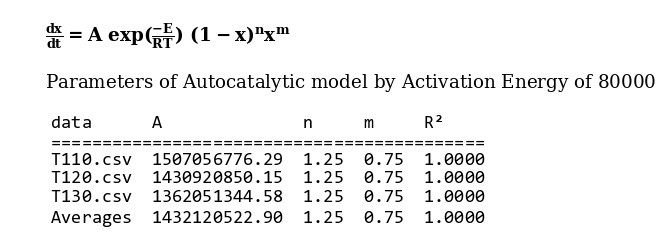
The amount of 80000 is considered as average value of activation energy this material. also calculating of other kinetics parameters of this sample by autocatalytic model is calculated by “MeasSoft Thermal Kinetics” Software as:
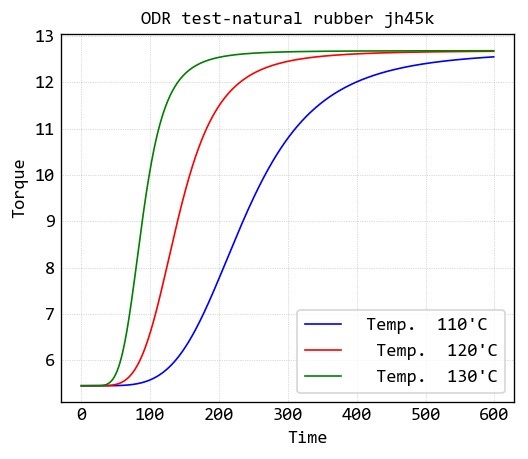
Static ODR of a natural rubber
For study of curing reaction of a compound of natural rubber (jh45k), Static ODR test of it at three heating rate of 10, 15 and 20 ̊C/min is given in below:
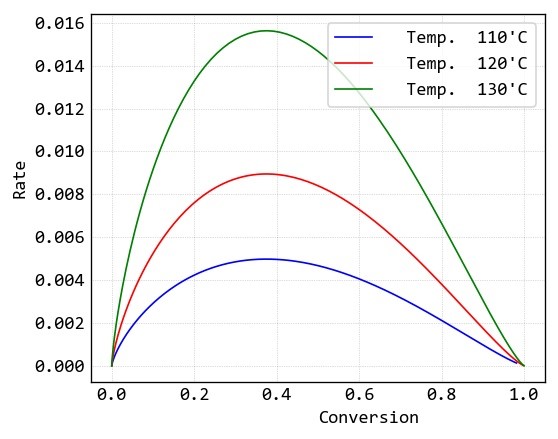
Conversion and reaction rate data of this samples are calculated by “MeasSoft Thermal Kinetics” Software as:
By helping “MeasSoft Thermal Kinetics” Software we get activation energy of this compound in isoconversional and ASTM methods as:
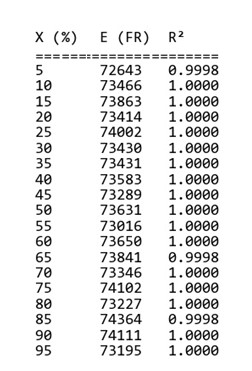

The amount of 73500 is considered as average value of activation energy this material. also calculating of other kinetics parameters of this sample by autocatalytic model is calculated by “MeasSoft Thermal Kinetics” Software as:
For buying "MeasSoft Thermal Kinetics" please contact us by:
This email address is being protected from spambots. You need JavaScript enabled to view it.


Write your comment